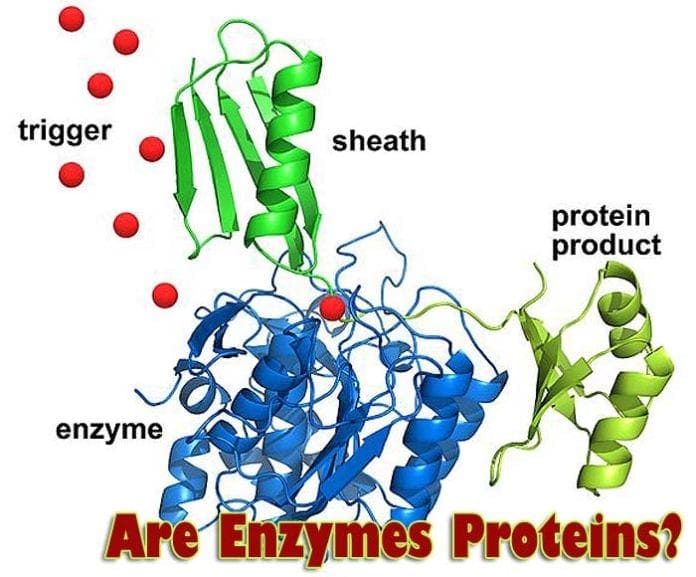
Are Enzymes Proteins? There are multiple processes taking place in our bodies. They are possible because of enzymes. These complex molecular structures perform multiple functions. Without them, many complex processes, starting from the creation of DNA itself, would be non-existent.
Let us look at them closer.
Table of Contents
Are Enzymes Proteins?
Yes. Basically, enzymes are a group of proteins that are able to catalyze reactions. Not all proteins have this property: there are structural proteins, or transport proteins, for instance.
Lately, it has been found that some other molecules too have an ability to speed up chemical reactions, but, as a rule, only catalytic proteins are called enzymes by scientists.
![]()
Are Enzymes Amino Acids?
No, enzymes are not amino acids. Amino acids are relatively simple organic molecules. Enzymes, however, are very complex. They are what chemists call polymers. They are molecules that are composed of multiple building blocks that can make long chains.
![]()
What Are Enzymes Made Of?
Enzymes are made of protein chains. These chains, in their turn, are formed by different amino acids. There are 21 amino acids that can be used by the human body to build proteins, and they can be combined in multiple ways. Some enzymes can consist of only one protein, while other enzymes can consist of multiple proteins.
There is also some evidence that RNAs also can work similar to enzymes, but most enzymes are proteins, and we will describe protein-based enzymes here.
![]()
Where Are Enzymes Found?
Enzymes can be found both inside and outside the cell. There are also antimicrobial and digestive enzymes in saliva, as well as digestive enzymes in secretions produced by the pancreas. Enzymes can be also found in the bloodstream in case of stress or damage to the heart or the liver.
![]()
What Are Enzymes Active Sites?
The proteins that work as enzymes are not simple chains. They usually are folded up into complex shapes. The shapes they form are essential. Each protein has a particular area that can participate in reactions with other molecules.
If you look at a wholly formed protein in 3D, it would be the hollow or cleft -like area composed of polar or non-polar amino acids that can specifically interact with a minimal number of molecules. This area is called the active site of an enzyme.
![]()
What Properties Do Enzymes Have?
Enzymes have several properties:
1. Enzymes form colloids
Colloids are gel-like substances. The Cytoplasm of the cell is a colloid made up of different molecules, for example. Due to that property, they are relatively stable chemically and can be isolated with special methods, such as dialysis.
![]()
2. Enzymes are sensitive
Most enzymes are:
- Sensitive to temperature. They are unable to work if the temperature is lower than a certain threshold, and they get destroyed if the temperature is above 42°C.
- Sensitive to pH. The cell usually has individual compartments with a certain pH, where only specific enzymes can work.
- Sensitive to inhibitory molecules. Specific molecules can interact with the active site of the protein and prevent it from supporting chemical reactions. An inhibitor is like a cork in a bathtub preventing the water from flowing out.
![]()
3. Enzymes are catalytic
Catalytic means that enzymes can significantly speed up reactions. For example, if you have a solution with two molecules that can potentially react with each other, the addition of the enzyme can speed up reaction from 108 to 1012 times compared to the usual rate.
![]()
4. Enzymes are highly specific
Each type of enzyme works with only one type of reaction or molecule. For example, enzyme lactase breaks up lactose and cannot react with anything else.
![]()
5. Some enzymes need co-factors
Cofactors are non-protein molecules, often elementary (for example, zinc). Without them, the enzymes are unable to participate in reactions.
![]()
How do enzymes work & speed up chemical reactions?
The ability of the enzyme to perform its function depends on its active site. The active site is formed by the residues of amino acids in such a way that it can bind to a molecule of a particular composition and shape. This mode of action is called the lock-and-key model.
- At present, there is another model that is called “induced fit model“. According to that idea, the enzyme reacts to the nearness of the correct molecule (called a substrate) and shapes its active site so the substrate would fit fully into it.
- The active site may have one or more “landing points” for molecules. This way, the enzyme can bring two molecules together so that they can react with each other.
If those molecules were just placed in the solution, they would react much slower. It happens for several reasons:
- First of all, each reaction requires a certain amount of energy. When the enzyme binds to the molecules participating in the reaction, it also lowers the amount of energy that would be required.
- During each reaction, the potential result product has to go through the so-called transition state. It is very unstable. However, when the molecules are bound to the enzyme, this transitional product is much more stable so that the reaction can go faster.
- The enzyme also weakens the bonds between the components of the molecule, so that it can be broken up or new parts can be attached to it.
When the reaction is finished, the products no longer fit into the active site of the enzyme, so they are released. Another molecule can take its place, and the whole process can begin again.
Inhibitors that can block the enzymes have similar shapes to the “correct” molecules, but they bind to the active site in the slightly wrong way so that the specific activity of this particular protein (for example, ability to attach chemical groups) is blocked.
![]()
What functions do enzymes perform in the cell?
There are several types of enzymes:
- Helicases – can unwind DNA;
- Ligases – join two separate molecules;
- Oxido-reductases – facilitate the electron transfer;
- Hydrolases – add -OH groups;
- Transferases – move a group from one molecule to another;
- Lyases – split chemical bonds;
- Isomerases – create isomers – molecules with the same composition, but another orientation;
In the natural environment, enzymes are responsible for digestion and creation of new molecules (nucleic acids, lipids, and other proteins).
They can also modify and control the activity of other molecules. Humans also use enzymes in the industry (Example: Washing powders).
![]()
Enzymes are crucially important for our well-being. For example, when the system of digestive enzymes fails, we can get severely sick, as we are unable to take up nutrients from our food. Because of their importance, enzymes are an active focus of researchers. There are still many things we do not know about them.
![]()
Cite This Page
Key References
- “Enzymes Chemistry, Polymers origin, Enzymes and Polymers Properties, Structures, Production, Industrial applications”. Accessed July 04, 2019. Link.
- “Book: Organic Chemistry with a Biological Emphasis (Soderberg) – Chemistry LibreTexts”. Accessed July 04, 2019. Link.
- “What are Ribozymes?”. Accessed July 04, 2019. Link.
- “Digestive enzymes — Science Learning Hub”. Accessed July 04, 2019. Link.
- “Chapter 3: Enzymes: Structure and Function” – CPP.edu. Accessed July 04, 2019. Link.
- “Enzymes: Properties and Mechanism of Enzyme Action”. Accessed July 04, 2019. Link.
- “Enzymes: Function, definition, and examples”. Accessed July 04, 2019. Link.
- “Structural Biochemistry/Specific Enzymes and Catalytic Mechanisms/Enzyme Classification – Wikibooks, open books for an open world”. Accessed July 04, 2019. Link.
- “File:Biochemical Protein Purification Challenge.jpg – Wikimedia Commons”. Accessed July 04, 2019. Link.