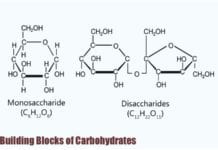
Building Blocks of Lipids: Living organisms are made of biomolecules (biological molecules) that are essential for performing physiological functions: carbohydrates, proteins, lipids, and nucleic acids. These molecules vary in size, structure, properties, and functions in and among cells.
Generally, their structures dictate their biological function. If by any chance the structure is disrupted or distorted, this could result in the impairment of the biomolecule itself.
Among these four biomolecules, lipids are considered to be unique as they are not defined by the presence of overall structural properties. Lipids are known for their hydrophobic or “water-fearing” properties that are due to the characteristics of their building blocks: glycerol and fatty acids.
In this article, explore the building blocks of lipids as well as how they are grouped together in order to form a lipid. Let’s take a closer look.
Table of Contents
Building Blocks of Lipids
Structure of A Lipid
Like any other biomolecules, lipids are made up of building block monomers. In biochemistry, a monomer refers to a single molecule that when chemically combined with other monomers (can be of the same type or other molecules) can form larger and different molecules. Basically, monomers are just composed of simple elements.
- Unlike the three biomolecules, lipids are not made up of “true” polymers because of their relatively smaller size and non-repeating monomers.
- As alluded to earlier, a lipid molecule is composed of a glycerol and (three) fatty acid sub-units. They are described in the following.
![]()
1. Glycerol
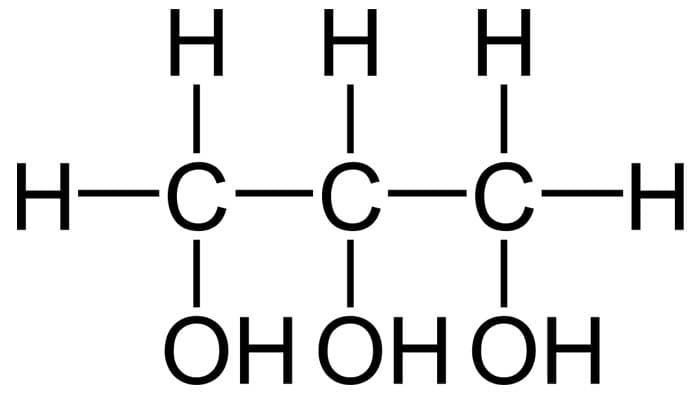
Considered to be a naturally occurring three-carbon alcohol (contains one carbon molecule that is bonded to three OH groups), glycerol is a molecule that serves as the structural backbone of a lipid. Aside from that, glycerol is also used to store energy.
- Because of its OH group, glycerol can be considered as a “polyol “, a type of alcohol that contains more than one OH group. Because of this property, glycerol can be readily dissolved in water.
- Additionally, the presence of these OH groups contributes to the hygroscopic property of glycerol. In other words, it can readily take up and retain water molecules.
- In layman’s term, glycerol is also known as glycerin or glycerine. In industries, glycerol is used as sweeteners and humectants.
![]()
2. Fatty Acids
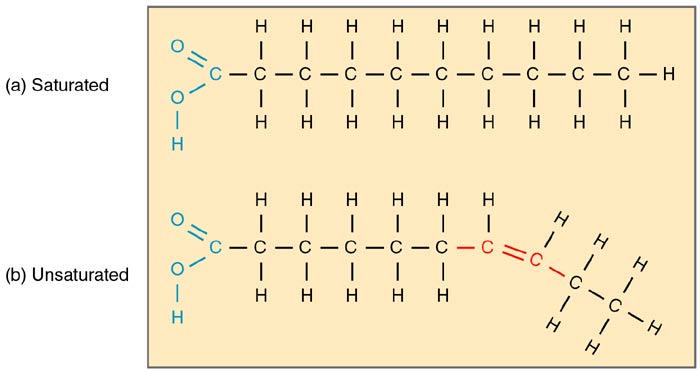
Fatty acids are chains of hydrocarbons that have various lengths and levels of unsaturation that end with carboxylic acid functional groups. The biochemical name of a fatty acid originates from the name of its parent hydrocarbon, with the final “e” being changed to “oic” and adding “acid” in the end.
- In biological systems, most fatty acids have an even number of carbon atoms, usually ranging from 14 to 24, with 16 and 18 carbon atoms being the most common. In animals, the hydrocarbon chain is always unbranched.
- The biochemical properties of fatty acids and their lipid derivatives are dependent mostly on the length of their chains and levels of saturation. As compared with their saturated counterparts (of the same length), unsaturated fatty acids tend to have lower melting points.
- In addition to this, the length of the chain also affects melting point because shorter chain lengths somehow affect the level of saturation and contribute to their fluidity.
- As compared with glycerol, fatty acids, being “fats” supply a relatively higher amount of energy per gram and have more biological roles than glycerol.
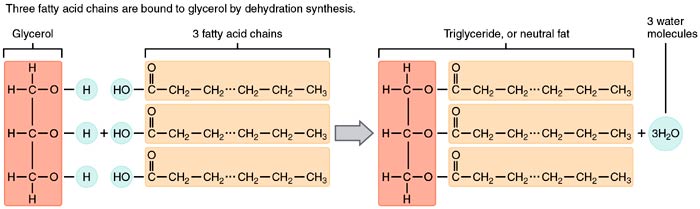
So how are these molecules combined and linked to form a lipid? The OH group found in the glycerol molecule and the carboxyl group of the fatty acids are covalently linked via an ester linkage. dehydration synthesis is needed in order to create this.
![]()
Examples of Lipids
There are actually quite a number of examples of lipids in biological systems. Below you can find the two most common and naturally occurring ones: cholesterol and triglycerides.
A. Cholesterol
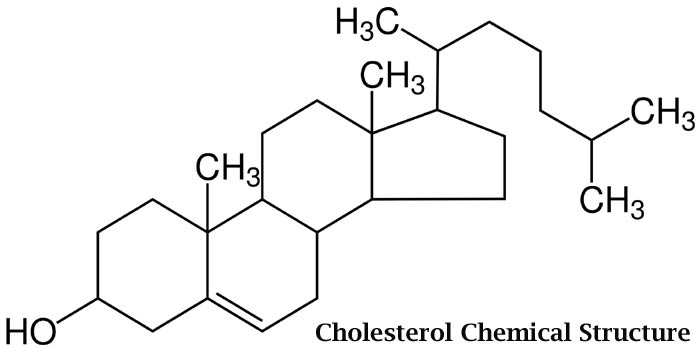
With a molecular formula of C27H45OH, you can see that the lipid derivative cholesterol is made up of three parts: a hydrocarbon tail, a hydroxyl group, and four hydrocarbon rings. Because of these structures (having fat soluble and water soluble regions, cholesterol is considered to be an amphipathic molecule.
- In living organisms, cholesterol serves as an important component of the cell membranes that enable the body to biochemically absorb fats and other fat derivatives like vitamins. Aside from that, cholesterol is used to synthesize vitamin D and hormones (i.e. cortisol, testosterone, and estrogen).
- While the body can naturally synthesize its own sources of cholesterol, it still needs to obtain it from other sources like food. Both synthesized and dietary cholesterol is transported in the body using lipoprotein molecules.
- Cholesterol is a type of lipid derivative that is based on steroids.
![]()
B. Triglycerides
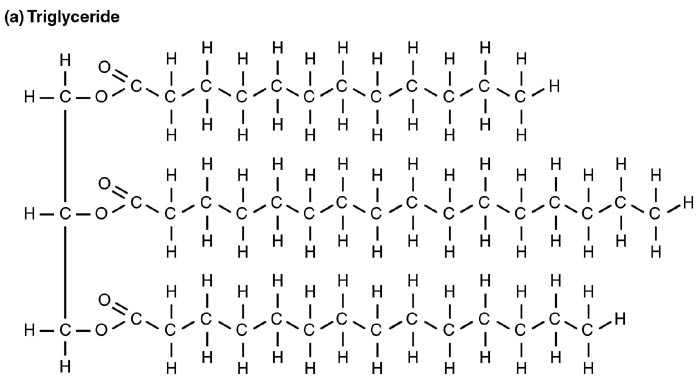
On the other hand, triglycerides are lipid derivatives that are derived mostly from glycerol. And as their name suggests, they are composed of three molecules of glycerols. Because most lipids are insoluble in water, they needed to be transported along with other molecules like proteins during circulation in the body.
- In animals, triglycerides are synthesized in the intestines and liver from fatty acid units. Contained in the fat cells, triglycerides are broken down into smaller units to provide the body with energy.
- To avoid toxicity and other harms, fatty acids are transported as triglycerides, and molecules like lipoproteins play a huge role in this process (i.e., transporting lipids to the peripheral tissues from the liver and back).
Aside from those above two, other examples of lipid derivatives include vitamins (those fat solubles one such as vitamins A, D, E, and K), and waxes. Fats, both the saturated (with single bonds) and unsaturated (with double bonds) ones, are also considered as lipid derivatives.
![]()
Functions of Lipids
Being nonpolar and hydrophobic as they are, lipids serve as strategic components of the plasma membrane and other cellular constituents like the nuclear membrane and envelope, endoplasmic reticulum (ER), Golgi body, lysosomes, and vesicles.
- Interestingly, the composition of these organelles mentioned above varies significantly, therefore suggesting that different types of lipids are needed for various biological functions.
- Like carbohydrates, lipids are also used for the storage of energy. However, the former is only used for immediate purposes while the latter serve for the long term.
- Furthermore, lipids also serve important roles in maintaining the structural integrity of organisms as well as exhibiting functions during cell signaling.
While a lot of people use the term “fats” for lipids interchangeably, it is important to note that the former is just actually a subgroup of the latter. Because of this notion, lipids are given the idea of having a negative role in health.
![]()
Despite their functions as mentioned earlier and biological importance, lipids have not been well researched as compared with the remaining biomolecules. The reason behind this is pretty simple: scientists and researchers think that fats are too complicated to work with (their nature and physiology), and the apparent lack of techniques in order to observe and visualize the levels of lipids.
Nevertheless, the recent advances and discoveries about the identification, structural properties, and biophysics of lipids suggest that this field of study has a lot more to show, thus encouraging broader exploration of these molecules.
![]()
Cite This Page
References
- Biomolecules – Accessed November 28, 2017. PDF
- “Structure and Function of Lipids – Video & Lesson Transcript | Study.com”. Accessed November 28, 2017. Link.
- “Fatty Acids Are Key Constituents of Lipids – Biochemistry – NCBI Bookshelf”. Accessed November 28, 2017. Link.
- “Overview of Cholesterol and Lipid Disorders – Hormonal and Metabolic Disorders – MSD Manual Consumer Version”. Accessed November 28, 2017. Link.