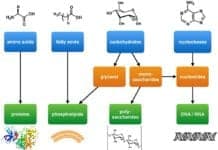
Dehydration Synthesis: Despite being very diverse, life can still be broken down into its 4 major building blocks: carbohydrates, proteins, lipids, and nucleic acids.
Being a constituent of living organisms, a more general name for this group of organic compounds is biomolecules. These biomolecules are needed for survival: carbohydrates and lipids for energy source, proteins for structural support, and nucleic acids for carrying genetic information.
Water is essential in numerous cellular processes. In fact, in the presence of water, dehydration synthesis and hydrolysis are the biochemical processes that are used to either build or break down the said biological molecules.
Let’s take a look and explore what actually happens in these reactions.
Table of Contents
What is Dehydration Synthesis?
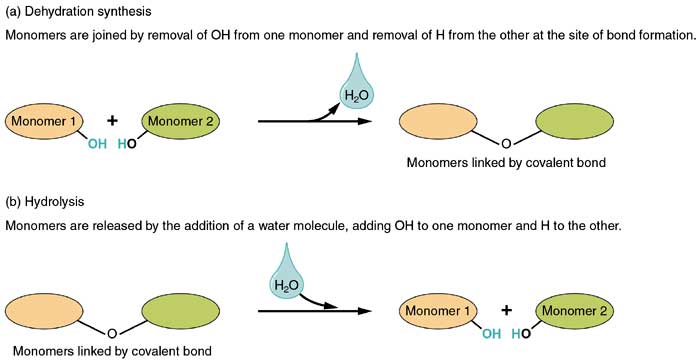
- In this process, a hydrogen ion (H+) from one component and a hydroxide ion (OH-) from the succeeding component are removed. The hydrogen ion and hydroxide then combine to form water, hence, the components appear to “lose water” or “dehydrate“.
- Dehydration synthesis is also called as “condensation reaction” because both are characterized by the condensation and formation of water from the large molecule.
- Examples of dehydration synthesis reactions are the conversion of monosaccharides to complex sugars, production of proteins from amino acids, conversion of fatty acids to complex fats, and the formation of nucleic acids from nucleotides.
The diagram below illustrates how dehydration synthesis generally occur in polymers.
![]()
Types of Dehydration Synthesis
In biological organisms, various types of dehydration synthesis occur. Such are classified based on the following:
1. Based on the nature of reactants
2. Based on the nature of the catalyst
3. Based on the product formed
![]()
What is Hydrolysis?
- Large molecules are broken down by breaking the bond between water molecules. In this process, a hydrogen ion (H+) is added to one component and a hydroxide ion (OH-) is added to another one.
- Since water is split, it is termed as “hydrolysis” which literally means “water separation“[3].
- Examples of hydrolytic reactions are the breaking down of complex sugars, proteins, complex fats, and nucleic acids into monosaccharides, amino acids, fatty acids, and nucleotides.
![]()
Types of Hydrolysis
Various types of hydrolysis occur in living organisms. The three types[4] are listed below:
1. Salt Hydrolysis
2. Acid Hydrolysis
3. Base Hydrolysis
![]()
Reactions of Dehydration Synthesis and Hydrolysis
| Type of Organic Compound | Type of Bond Involved | Dehydration Synthesis | Hydrolysis |
|---|---|---|---|
| Carbohydrates | Glycosidic bond | Two or more monosaccharides combined = Disaccharide/Polysaccharide + H2O | Disaccharide/Polysaccharide + H2O = Monosaccharide units |
| Proteins | Peptide bond | Two or more amino acids =Dipeptide/Protein + H2O | Dipeptide/Protein + H2O = Two or more amino acids |
| Lipids | Ester bond | 3 Fatty acids + Glycerol = Lipid molecule | Lipid molecule + 3 H2O = 3 Fatty acids + Glycerol |
| Nucleic acids | Phosphodiester bond | 10 nucleotides = Nucleic acid + H2O | Nucleic acid + H2O = 10 nucleotides |
Should you need more illustrations, you may check this YouTube video below:
![]()
Roles of Dehydration Synthesis in Biological Systems & Examples
- Another common example of dehydration synthesis in plants is the production of maltose (malt sugar) from the fusion of two glucose units resulting from the freeing of water molecule. Other polymers of carbohydrates are also formed this way.
![]()
Roles of Hydrolysis in Biological Systems & Examples
- In animals, digestion is one of the most common example of hydrolysis. Food is broken down (hydrolyzed) into small units by different enzymes present in the digestive tract. Such pave the way for the easier absorption of nutrients in the small intestine.
- Other examples include the hydrolysis of fat as this helps in the prevention of them clotting in different parts of the body.
![]()
From being a mere thirst quencher to a facilitator of photosynthesis to becoming a builder or breaker of molecular compounds, we have seen that water is undeniably essential for living organisms at the molecular level. What do you think are other abilities and uses of this compound?
![]()